The BIOFIRE® SPOTFIRE® Respiratory/Sore Throat Solution
Multiplex PCR Testing Built for the Point of Care
An evolving world deserves evolved diagnostics. As the leader in syndromic PCR testing, bioMérieux has evolved our point-of-care respiratory testing solution by developing new products that put clinicians and patients front and center. bioMérieux is pleased to announce a revolutionary portfolio of versatile respiratory PCR tests that give clinicians options as they care for a variety of patients.


- Product Overview
- Technical Details
- Service & Support
- Resources
Product Overview
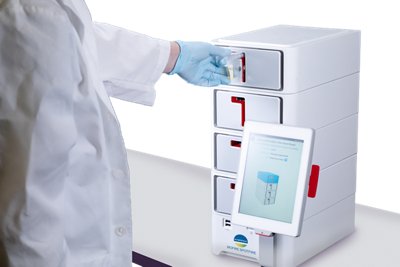
The BIOFIRE® SPOTFIRE® System
The SPOTFIRE System is the latest advancement in molecular infectious disease diagnostics. It is designed to run the SPOTFIRE Respiratory/Sore Throat Panels.
- Requires minimal benchtop space and can be scaled vertically, up to four modules, enabling customizable throughput
- Easy to use with an intuitive system interface and streamlined workflow
- Can be used by any healthcare professional in any healthcare setting
- Seamlessly connects with point-of-care data management systems and other compatible systems and software

1. Calculation based on running the BIOFIRE® SPOTFIRE® R PANEL (~15 minutes) in an 8 hour shift.
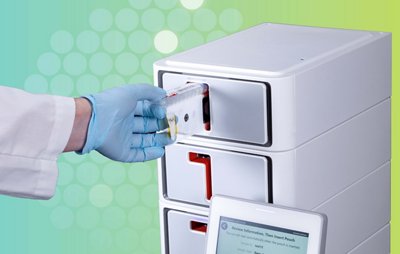
BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel
1 PCR Test. Up to 15 Targets. ~15 Minutes.
The SPOTFIRE R/ST Panel enables testing for both respiratory or sore throat pathogens on the same panel, providing a flexible respiratory solution to meet the unique needs of your care setting. FDA CLEARED | CLIA WAIVED
| Sample Type: | Nasopharyngeal or throat swab in transport media |
| Hands-on time: | 1-2 minutes |
| Overall Respiratory Performance (NPS specimens): | PPA: 98.5% NPA: 99.1% |
| Overall Sore Throat Performance (TS specimens): | PPA: 95.9% NPA: 99.0% |
| RESPIRATORY | SORE THROAT | |
|---|---|---|
| VIRUSES: | VIRUSES: | |
|
| |
| BACTERIA: | BACTERIA: | |
|
|
BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel Mini
1 PCR Test. 5 Targets. ~15 Minutes.
The SPOTFIRE R/ST Panel Mini tests for the 5 most probable viruses or bacteria that cause respiratory and sore throat symptoms, including influenza, rhinovirus, respiratory syncytial virus, SARS-CoV-2, and Streptococcus pyogenes.
FDA CLEARED | CLIA WAIVED
Panel Specifications
| Sample Type: | Nasopharyngeal or throat swab in transport media |
| Hands-on time: | 1-2 minutes |
| Overall Respiratory Performance (NPS specimens): | PPA: 98.7% | NPA: 98.4% |
| Overall Sore Throat Performance (TS specimens): | PPA: 96.2% | NPA: 97.9% |
| RESPIRATORY | SORE THROAT |
|---|---|
| VIRUSES: | VIRUSES: |
|
|
| BACTERIA: | |
|
Rethink the Power of Diagnostics with the SPOTFIRE Respiratory/Sore Throat Solution to Potentially:
Inform Clinical Decisions
- High sensitivity, specificity, and reliability provides results clinicians can trust
- Contrary to tests that only detect the flu, SARS-COV-2, and/or RSV, a syndromic panel provides a fuller picture that may help inform patient management decisions
Optimize Operations
- The simplified workflow and quick turnaround time enable rapid and accurate answers during the patient visit, while reducing the need for callbacks or expensive and slow send-out testing
- This easy-to-use solution enables users of all skill levels to prepare and run samples
Optimize Antimicrobial Stewardship
- Receiving comprehensive results in a clinically actionable timeframe may inform diagnoses while optimizing care and antimicrobial stewardship
Improve Patient Satisfaction
- Using the right test, the first time to detect multiple pathogens may result in reduced follow-up appointments, swabbing, and testing, which could improve patient comfort, experience, and convenience
Product availability varies by country. Consult your bioMérieux representative.
Reference:
- Calculation based on running the BIOFIRE® SPOTFIRE® R PANEL (~15 minutes) in an 8 hour shift.
- Overall performance and SARS-CoV-2 performance based on the prospective clinical study for the BIOFIRE SPOTFIRE Respiratory Panel.
Technical Details
SPOTFIRE Respiratory Panel:
- Sample Type: Nasopharyngeal swab in 1-3 mL of viral transport media
- Hands-on time: 1-2 minutes
- Overall Peformance:1 PPA: 98.5%, NPA: 99.1%
- SARS-CoV-2 Performance:1 PPA: 97.3%, NPA: 99.4%
SPOTFIRE Respiratory Panel Mini:
- Sample Type: Nasopharyngeal swab in 1-3 mL of viral transport media
- Hands-on time: 1-2 minutes
- Overall Performance:1 PPA: 98.7%, NPA: 98.4%
- SARS-CoV-2 Performance:1 PPA: 97.3%, NPA: 99.4%
SPOTFIRE Respiratory Panel Menu
VIRUSES:
- Adenovirus
- Coronavirus SARS-CoV-2
- Coronavirus (seasonal)
- Human metapneumovirus
- Human rhinovirus/enterovirus
- Influenza A virus
- Influenza A virus A/H1-2009
- Influenza A virus A/H3
- Influenza B virus
- Parainfluenza virus
- Respiratory syncytial virus
BACTERIA:
- Bordetella parapertussis
- Bordetella pertussis
- Chlamydia pneumoniae
- Mycoplasma pneumoniae
SPOTFIRE Respiratory Panel Mini Menu
VIRUSES:
- Coronavirus SARS-CoV-2
- Human rhinovirus
- Influenza A virus
- Influenza B virus
- Respiratory syncytial virus
Product Information
| PRODUCT NAME | PART NUMBER | QUANTITY |
| BIOFIRE SPOTFIRE Respiratory Panel Kit | 424461 | 30-Pack |
| BIOFIRE SPOTFIRE Respiratory Panel Mini Kit | 424589 | 30-Pack |
| SPOTFIRE RSP Pos & Neg Controls | M425 | 2 boxes (1 x M42638, 1 x M42738) |
| SPOTFIRE RSP Positive Controls | M42638 | 1 box (6 tubes) |
| SPOTFIRE RSP Negative Controls | M42738 | 1 box (6 tubes) |
| NATtrolTM Respiratory Verification Panel | NATRSP-BIO | 1 Panel |
| NATtrol™ Respiratory Verification Panel Mini | NATRMP-BIO | 1 Panel |
| BIOFIRE SPOTFIRE Respiratory Panel, Verification Kit | 424595 | 1 x NATRSP-BIO, 5 x M425, 3 x 424461 |
| BIOFIRE SPOTFIRE Respiratory Panel Mini, Verification Kit | 424596 | 1 x NATRMP-BIO, 5 x M425, 3 x 424461 |
| BIOFIRE SPOTFIRE Control Station | FAST-ASY-0001 | 1 |
| BIOFIRE SPOTFIRE Module | FAST-ASY-0002 | 1 |
Service & Support
With the urgent nature of patient and community care, we take pride in addressing all concerns quickly and accurately. bioMérieux is dedicated to providing world class customer support 24 hours a day, 7 days a week, 365 days a year.
For assistance please contact our customer technical support team at:
- Email: biofiresupport@biomerieux.com
- Phone: 801-736-6354 option 5
- Toll Free: 1-800-735-6544
To order, contact your regional sales representative or use the following contact information:
- Phone: +1-801-736-6354 x 1502
- Fax: +1-801-588-0507
- Email: CSCFax@biomerieux.com
- International Sales: +1-801-736-6354 x 1536
- Military Sales: 1-801-262-3592